Are you sourcing sweeteners and find sodium saccharin confusing? It's called a salt but used for sweetness, which raises questions. Let's clarify what this common additive is and how it's made for sourcing.
Sodium saccharin is the salt form of saccharin, an artificial, non-nutritive sweetener. It's produced through chemical synthesis, typically starting from raw materials like toluene or phthalic anhydride via processes like the Remsen-Fahlberg or Maumee methods.
Knowing its composition, sweetness level, and production methods helps you make informed decisions for your business. Let's break down these key aspects.
What Is the Chemical Composition of Sodium Saccharin?
Confused about what sodium saccharin actually is chemically? Knowing the specific makeup helps ensure you're sourcing the right, high-purity ingredient. Let me outline its precise structure.
Sodium saccharin is the sodium salt of benzoic sulfimide. Its chemical formula is C7H4NNaO3S. It exists mainly as sodium saccharin dihydrate in its commercial form, meaning it includes two water molecules.
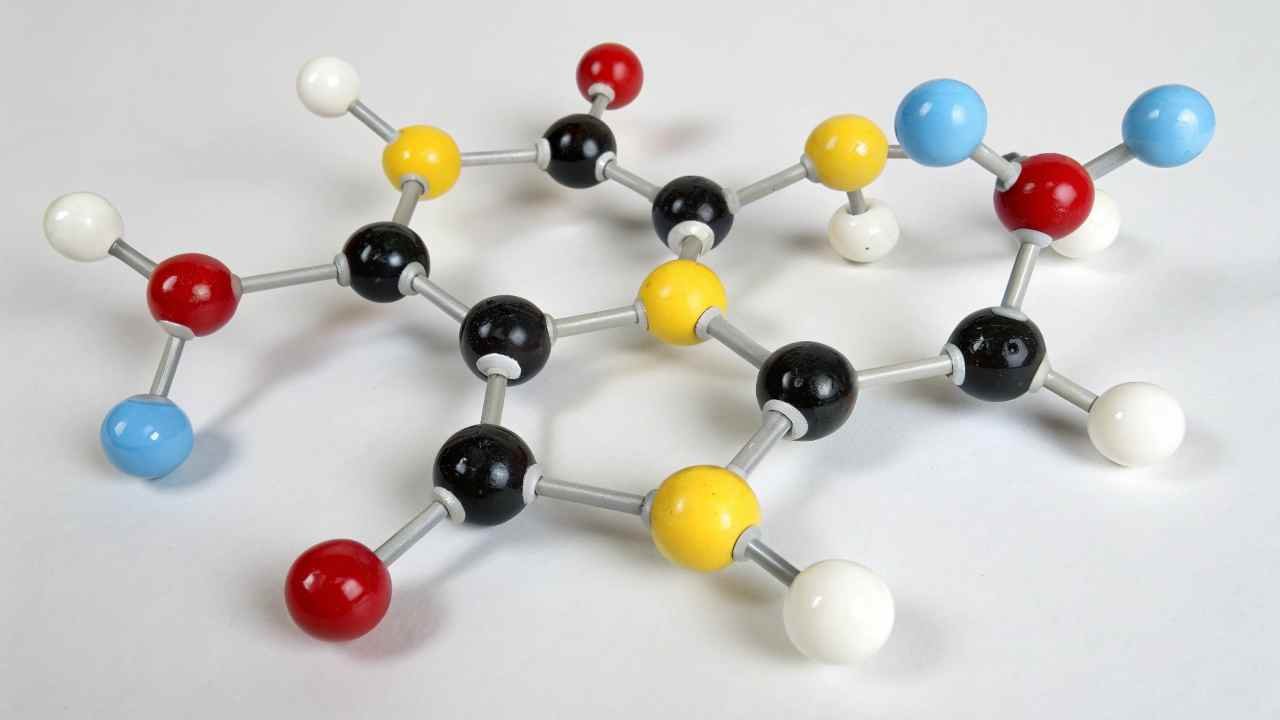
Key Chemical Points
The core molecule (C7H4NO3S-) provides the sweetness. Adding sodium (Na+) makes it a salt and, crucially, makes it much more soluble in water than saccharin acid1 alone. This better solubility is why the sodium form2 is preferred industrially. Most commercial products are the dihydrate (C7H4NNaO3S · 2H2O). Specification sheets, like those we provide at FINETECH, confirm the form and purity, which are vital quality checks.
How Many Times Sweeter Is Sodium Saccharin Compared to Sugar?
Wondering just how potent sodium saccharin is? Knowing its sweetness intensity is key for correct usage levels3 and cost calculations in your formulations. Let’s establish its relative sweetness.
Sodium saccharin is approximately 300 to 500 times sweeter than regular table sugar (sucrose) by weight. The exact perceived sweetness can vary depending on concentration and the food matrix it's used in.

Sweetness Implications
This high intensity means you need only a tiny amount, leading to significant cost savings and making it ideal for low-calorie or sugar-free products. While potent, a slight metallic aftertaste4 can occur at high concentrations, which is often managed by blending it with other sweeteners. Its intensity-to-cost ratio is a major reason clients choose it.
Why Is Sodium Saccharin Classified as a Salt but Used as a Sweetener?
Confused by the terminology? Hearing "sodium salt" might make you think of table salt, not a sweetener. Let's clear up why it has this chemical classification but functions differently.
Sodium saccharin is chemically a salt because it's formed from the reaction of an acid (saccharin acid/benzoic sulfimide) with a base containing sodium (like sodium hydroxide). However, the saccharin part (anion) triggers sweetness receptors on the tongue.

Chemistry vs. Function Explained
In chemistry, "salt" means an acid reacted with a base. Here, saccharin acid + sodium hydroxide -> sodium saccharin + water. The sweetness comes from the shape of the saccharin molecule fitting taste receptors. The main reason for using the sodium salt form isn’t taste, but vastly improved solubility5 in water compared to saccharin acid, making it much easier to use in food and beverage production.
Where Does Sodium Saccharin Originate from Industrially?
Curious about the manufacturing roots of sodium saccharin? Knowing the typical starting materials and processes helps in assessing supplier capabilities and potential quality variations. Let's look at its industrial synthesis.
Industrially, sodium saccharin is primarily produced through chemical synthesis starting from petrochemical feedstocks. The two main historical methods are the Remsen-Fahlberg process (starting from toluene) and the Maumee process (starting from phthalic anhydride).

Production Hubs and Quality Control
China is currently one of the largest producers of sodium saccharin globally. Regardless of the method used, stringent quality control throughout the process is essential. This involves:
- Testing raw materials.
- Monitoring reaction conditions (temperature, pressure, reaction time).
- Purification steps6 (like recrystallization) to remove impurities.
- Final product testing against specifications (assay, moisture, heavy metals, specific organic impurities).
At FINETECH, we partner with manufacturers who demonstrate robust quality management systems to ensure the sodium saccharin we supply consistently meets international standards required by clients in Europe, the Middle East, and Southeast Asia.
What Are the Primary Industrial Uses of Sodium Saccharin?
Wondering where sodium saccharin actually ends up? Knowing its main applications helps understand its market relevance and why consistent supply is important. Let's review its key industrial roles.
The primary industrial uses of sodium saccharin are as a non-caloric sweetener in foods (especially baked goods, jams, chewing gum), beverages (soft drinks), tabletop sweeteners, pharmaceuticals (masking bitter tastes), and even in non-food applications like electroplating.

Applications Across Industries
Sodium saccharin's unique properties – high sweetness intensity, stability (especially to heat), and low cost – make it versatile. Its applications span several sectors, which is why it remains a staple for many distributors and manufacturers I work with.
Here's a quick summary of why it's chosen for different uses:
| Application Area | Key Reasons for Use | Examples |
|---|---|---|
| Food & Beverage | High Sweetness, Heat Stability, Cost-Effective, Soluble | Diet Soda, Baked Goods, Jams, Tabletop Sweetener |
| Pharmaceuticals | Taste Masking (Bitter Drugs), Stability | Syrups, Chewable Tablets |
| Personal Care | Flavor Improvement | Toothpaste, Mouthwash |
| Industrial | Technical Properties (Brightener) | Nickel Electroplating |
This wide range of uses ensures continued demand for sodium saccharin, making reliable sourcing and quality assurance, like we provide at FINETECH, essential for businesses globally.
Conclusion
Sodium saccharin is the highly soluble, heat-stable sodium salt of saccharin acid, produced chemically. It's valued for its intense sweetness (300-500x sugar) and used widely in food, drinks, pharma, and industry.
-
Exploring this link will provide you with detailed insights into saccharin acid, its properties, and applications in various industries. ↩
-
Understanding the advantages of the sodium form can enhance your knowledge of its industrial applications and benefits over other forms. ↩
-
Knowing the correct usage levels ensures optimal flavor and cost-effectiveness in your formulations. Check this link for guidelines. ↩
-
Understanding the causes and management of metallic aftertaste can enhance product formulation and consumer satisfaction. ↩
-
Understanding improved solubility can help you grasp its critical role in food and beverage formulation, enhancing product quality. ↩
-
Explore this link to understand various purification techniques that enhance product quality and efficiency. ↩


